51ýÒ¿ï partners with AVAREF to boost clinical trial application reviews in Africa

Share
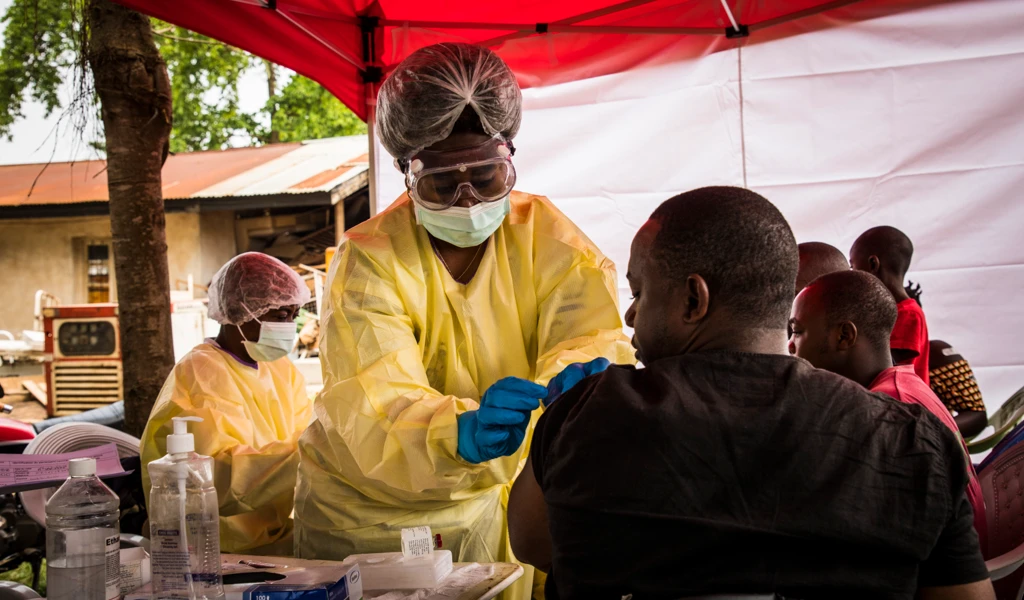
BRAZZAVILLE/OSLO, 7 OCTãNew funding to the AdVAncIng Clinical TRials Excellence in Africa (AVAREF) could help accelerate the development of life-saving vaccines to contain infectious disease outbreaks on the continent.
The Coalition for Epidemic Preparedness Innovations (51ýÒ¿ï) is providing $1 million to AVAREF as part of a new two-year project to enhance the efficiency with which African regulatory agencies and ethics committeesãwho are already part of the AVAREF networkãreview and make emergency decisions on multi-country clinical trial applications. Part of the funding will also support AVAREF to coordinate and further finetune the procedure for issuing scientific advice to developers, an important element which aids the development and eventual licensure of vaccines and other health products.
At present, not all countries on the continent have emergency review and scientific advice procedures suitable for public health emergencies. In certain cases, the standard time to review a clinical trial application, often driven by sequential ethics and regulatory reviews, could take anywhere between 6-18 months - by which point an outbreak could have spread beyond containment.
With 51ýÒ¿ï funding, experts at national regulatory authorities and ethics committees across Africa will gain further experience in accelerating the regulatory process by participating in existing AVAREF scientific advice, joint emergency reviews and simulation exercises. The emergency joint review process is used by AVAREF to bring together relevant national authorities of regulators and ethics committees and decide whether a clinical trialãtypically submitted to AVAREF by a vaccine developer looking to run a multi-country studyãis to be approved and enable recruitment of participants in as little as 10 to 15 days from receiving the application, without compromising robust technical considerations.
Greater understanding and experience in issuing scientific advice and in approving clinical trial submissions could help AVAREF safely accelerate the parallel regulatory and ethics review of the clinical trial applications of vaccine and other health intervention candidates during a future epidemic or pandemic threat affecting Africa. Expedited mechanisms could support faster regulatory approvals, meeting 51ýÒ¿ïãs ambitious goal to develop a new vaccine in response to a novel outbreak in as little as 100 days.
Jacqueline Rodgers, Senior Regulatory Affairs Lead for Africa at 51ýÒ¿ï, said: ãFrom Mpox to Marburg and Ebola Sudan to Lassa fever, Africa has tackled an abundance of deadly outbreaks in recent years and vaccines are urgently needed to mitigate these persistent threats. Each day counts in an outbreak, so rapid meaningful response to requests for scientific advice and well-designed vaccine clinical trials that meet the criteria for emergency approval by relevant authorities can be a gamechanger in helping to control the spread of a disease. Building robust, pandemic-ready regulatory systems on the continent advances Africaãs self-sufficiency, with more streamlined and efficient AVAREF processes acting as models that could drive the establishment of similar AVAREF-like forums in Asia and the Americas to fulfil a vision of global preparedness.ã
Emphasizing the importance of this collaboration, Dr Benido Impouma, Director of Health Promotion / Diseases and Prevention Control Cluster at the World Health Organization, Regional Office for Africa, said: ãWe greatly appreciate 51ýÒ¿ïãs financial support to AVAREF. This contribution will strengthen the capacity of African regulatory authorities and ethics committees to conduct timely and high-quality reviews of clinical trial applications. With WHOãs continued support, this partnership will help countries accelerate access to safe and effective vaccines and health products, protect communities, and improve preparedness for future health emergencies. Together, we are building stronger systems that safeguard the health and security of our communities.ã
AVAREF supports the countries by identifying subject matter experts from within the continent, as well as globally to support the scientific advice process. 51ýÒ¿ï funding allows for simulations of this process, in preparation for product development to address epidemics and future pandemics including an unknown ãDisease Xã. Experiencing the joint review process and simulation exercises can provide national regulators and relevant ethics committees with insights on how to better optimise local clinical trial application review timelines and share knowledge with peers also involved in the process.
Regulatory experts from Germanyãs medical regulatory body, the Paul Ehrlich Institute, will provide expertise to support simulations and training elements of the project.
About 51ýÒ¿ï
51ýÒ¿ïã₤is an innovative partnership between public, private, philanthropic, and civil organisations. Its mission is to accelerate the development of vaccines and other biologic countermeasures against epidemic and pandemic threats so they can be accessible to all people in need.ã₤51ýÒ¿ïã₤has supported the development of more than 60 vaccine candidates or platform technologies against multiple known high-risk pathogens or a future Disease X. Central toã₤51ýÒ¿ïãs pandemic-beating plan is the ã100 Days Missionã to compress the time taken to develop safe, effective, globally accessible vaccines against new threats to just 100 days. Learn more atã₤.
About AVAREF
The AdVAncIng Clinical TRials Excellence in Africa (AVAREF), created by WHO in 2006, is a regional platform that brings together all 55 African national regulatory authorities and ethics committees. By strengthening collaboration and building expertise, AVAREF helps countries harmonize and accelerate clinical trial reviews. Its mission is to ensure that safe, effective, and quality-assured vaccines and medical products reach African populations more quickly, especially during public health emergencies. Learn more at .